The American Chemical Society (ACS) Spring 2024 conference, held from March 17-21 in New Orleans, Louisiana, was a vibrant mix of hybrid, in-person, and live virtual sessions. The theme of the conference was “Many Flavors of Chemistry,” reflecting the diverse range of topics and research presented.
One of the standout attendees was Dr. Balázs L. Tóth. Dr. Tóth presented his work entitled, “Alkynyl cyclic carbonates as 1,3-enyne surrogates in photoredox / cobalt-catalyzed reductive coupling.”
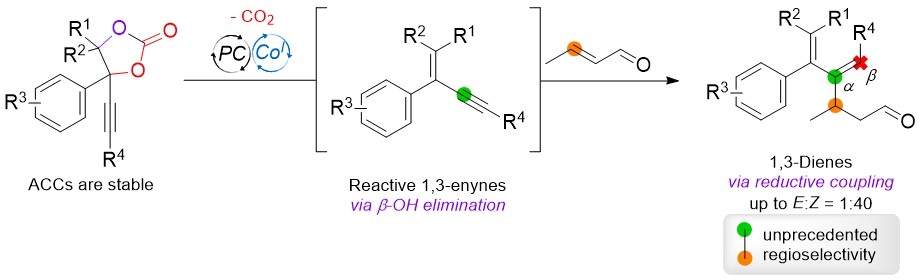
1,3-Enynes are important and versatile building blocks in organic synthesis as they are prone to be transformed into allene and 1,3-butadiene products. However, compared to simple alkynes they are more reactive and thus difficult to handle, whereas regioselective additions are challenging. Herein we present a dual Co/photoredox-catalyzed approach that utilizes modular and stable Alkynyl Cyclic Carbonates (ACCs) to generate in situ 1,3-enynes, which can be intercepted by crotonaldehyde in a novel reductive cross-coupling reaction. The double bond of the conjugated enyne intermediate is formed by a β-OH elimination step and remains inert in the presence of aldehyde reactants. However, under reductive reaction conditions the triple bond is favoured to undergo a Co-promoted formal hydroalkylation. Furthermore, this unprecedented stereo- and regioselective coupling reaction of 1,3-enynes is in contrast to the typical reactivity of internal alkynes that selectively provide the β-regioisomer.
The ACS Spring 2024 conference was an excellent platform for Dr. Tóth to share his groundbreaking work. His presentation not only added to the “Many Flavors of Chemistry” but also left a lasting impression on the attendees, further establishing his reputation as a researcher in his field.
